Identifying the business/technology issues and solutions for making clinical genomics standard of care – with a call to action

Adopting one ID for every uniquely offered
genetic test
- Share this post:
Introduction
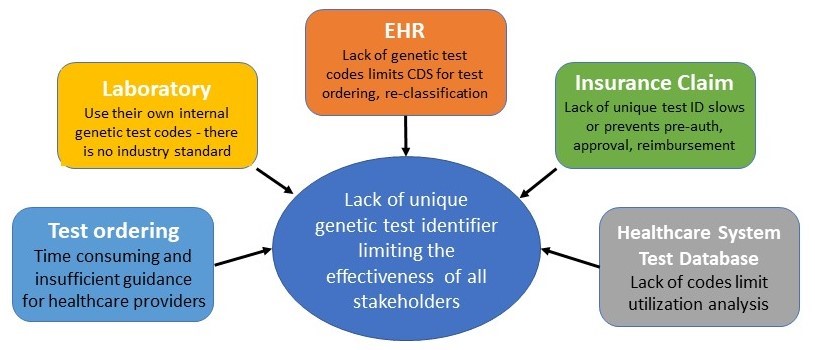
Every year the number of healthcare providers offering genetic testing services grows, including hospitals, private practices, academic medical centers, direct-to-consumer commercial companies, and other organizations. Currently, there are no widely accepted standards for defining what constitutes a “unique genetic test”. The lack of an ID for each unique genetic test raises the possibility of confusion among clinicians ordering tests and laboratories reporting them.
What is Needed
When using genetic testing for patient care, it is critical that all ordering clinicians and laboratories use the same standard to ensure accurate information exchange. This would then support the ability to report on the performance characteristics of each test in a consistent manner. The most logical conclusion here is to adopt a standard that uses a single identification code for each uniquely offered genetic test. This test coding standard is critical not only for improving genetic testing accuracy and efficiency, but also for improving the ability to perform clinical utility analyses for each test over time.
My Experience
Looking back more than 15 years, I was trying to perform utilization analyses on genetic testing for my employer, a large integrated healthcare system. The problem was that our lab results database used either generic or high-level codes for all of the genetic tests. Most tests were coded as “MSO” (miscellaneous send-outs), while others had high-level codes like “CFMUT” for any cystic fibrosis mutation test or “FRXDNA” for any Fragile X Syndrome test.
There are over 175,000 genetic testing products, ranging from single gene mutation tests and microarrays to multi-gene to whole exome and whole genome assays. We could not determine the clinical effectiveness of each test because we could not clearly differentiate between them. Compounding this issue was the fact that ICD-10-CM codes do not exist for many genetic mutations that are deleterious for the disease.
Laboratory issues and data standardization
Labs create similar but distinct tests as they compete for customers. Even among similar tests, differences in meta-data, read quality, bioinformatics approach, test methodology, and reference sequences used for interpretation contribute to the uniqueness of each test.
Data standardization is important because it eliminates the possibility of confusion among clinicians, laboratories, and patients when ordering tests. In the absence of a standardized method for identifying genetic tests offered by a laboratory, clinicians may use terminology other than that used by the lab to prescribe them. Laboratories frequently have multiple processes for identifying each genetic test offered, depending on how they want their customers (clinicians) or other stakeholders (patients) to interact with that information.
Solving the issue for all stakeholders (doctors, patients, EHR systems, labs, payors)
Here are some of the cross-stakeholder issues that the adoption of the GTU identifier would solve:
- The ability to search and compare similar tests from different labs, and determine if the test is clinically appropriate for the patient.
- A unique genetic test ID, when linked to details about the test, would be key to developing advanced genetics-based clinical decision support (CDS).
- Help healthcare providers navigate ordering and understand the significant differences in genetic test coverage between health plans.
- Help standardize pre-auth and billing policies for genetic testing among the various health insurance plans.
- Advance the quality and volume of genetic test assessments and medical necessity policies.
Perspective Report
A comprehensive review of this post
Factsheet Summary
A one-page summary of the post’s major issues, including executive highlights
The Call to Action
Concert Genetics has a solution, the Genetic Testing Unit (GTU). The GTU is a five-digit alphanumeric character sequence for every uniquely marketed test, even after the test is no longer available. I propose that the industry adopt this coding system as a standard today – so we can move forward. To participate in this effort, join us at Call-to-Action #5.
The industry’s use of the GTU identifier coding system will complement the use of CPT and HCPCS procedure codes, eliminate claim field ambiguity, reduce administrative work and costs, and most importantly, help expand patient access to genetic testing and precision therapies.
Insurance plan issues
The Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) have approximately 670 codes to represent the 175,000 tests when an insurance claim includes a genetic test. A widely used identifier would solve efficiency problems by eliminating uncertainty, which leads to manual reviews, incorrect payments, or denial of genetic testing services on the front end, and makes it difficult to determine the economic and clinical value of genetic testing through data analysis on the back end.
Updating X12 EDI standards for health insurance claims
To support and promote GTU adoption, an updated ASC X12 837 standard has been submitted. The proposal will:
- Authorize the use of the K3 segment on the 837P claim transaction for the GTU genetic test identifier, complementing the most appropriate HCPCS code(s).
- Engage X12 EDI standards development about authorizing the use of segments on other X12 health care transactions (e.g., 278 referral/auth, 835 remittances).
If you have questions or comments for X12, you can provide feedback here.
The GTU Adoption Outlook
Concert Genetics has made the GTU available for use royalty-free, and will only require a license for derivative works. The business evidence proving the validity of this proposal includes the following:
- All key requirements and criteria for a genetic test ID are met by the GTU standard.
- The GTU is compatible with existing integration methods and systems.
- The GTU does not conflict with other classification and identification systems already in use.
- IDs are assigned as soon as new tests are released to the public.
- In addition, six health plans have already requested that GTU codes be added to the Procedure Description element (837P SV101-7) of their published payment policies. Four more health plans will join them in the next few months, with many others starting the adoption process.
Conclusion – Opportunities for Moving Forward
The time for endless contemplation and indecision on this issue is over. It’s time to implement the GTU identifier and move forward. For those who agree, report back to me on your progress, and I will cover your organizational achievements in this blog. Labs and health plans that want to incorporate the GTU into their billing systems and practices should email info@concertgenetics.com.
Contact Grant at moveforward@genomics.network.

